A November 2024 meta-analysis published in Stem Cell Research & Therapy examining seven controlled human trials found that stem cell therapy produced significant improvements in motor nerve conduction velocity (WMD: 2.2) and sensory nerve conduction velocity (WMD: 1.9) among patients with diabetic peripheral neuropathy. For the over 615,000 Arizona adults living with diabetes—many of whom will develop debilitating nerve damage—these findings represent genuine hope. This article explores how Arizona Pain and Spine Institute applies regenerative medicine principles to treat neuropathy, the science behind stem cell therapy for nerve pain, and what Arizona residents can expect from this innovative treatment approach.
What Is Stem Cell Therapy for Neuropathy?
Peripheral neuropathy affects the nerves outside the brain and spinal cord, causing symptoms ranging from tingling and numbness to severe burning pain. According to data published in Scientific Reports from Johns Hopkins University researchers, the prevalence of peripheral neuropathy reaches 10.4% among middle-aged adults (40-69 years) and climbs to 39.2% among adults over 70 years old.
At Arizona Pain and Spine Institute, Dr. Asim Khan and Dr. Daniel Ryklin have been applying regenerative medicine approaches to chronic pain conditions since 2016. The practice uses amniotic stem cells ethically sourced from healthy donors following stringent FDA protocols. These cells contain natural growth factors that promote tissue regeneration and reduce inflammation at the cellular level.
Dr. Khan, who holds double board certifications in Physical Medicine and Rehabilitation and Pain Management, explains the approach on the practice’s website: “By combining innovative therapies with proven treatments, we can address both the symptoms and the underlying causes.”
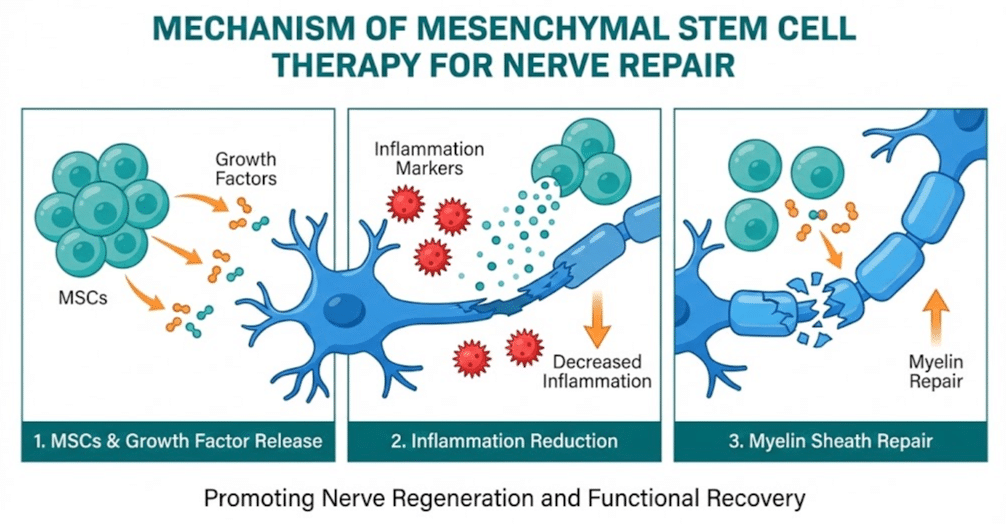
Unlike traditional neuropathy treatments that primarily mask symptoms through pain medications, stem cell therapy aims to support the body’s natural repair mechanisms. The mesenchymal stem cells (MSCs) used in regenerative treatments have demonstrated the ability to reduce inflammation, promote the healing of damaged tissue, and stimulate the growth of healthy new cells.
Get Back Your Normal Life Again
As pain specialists, we can guarantee that we are more than qualified in alleviating your pain and treating your condition.
How Arizona Pain and Spine Institute Approaches Neuropathy Treatment
Arizona Pain and Spine Institute has developed a comprehensive approach to neuropathy care that combines diagnostic precision with regenerative treatments. The practice operates from multiple East Valley locations including Mesa, Gilbert, and Queen Creek, serving the greater Phoenix metropolitan area.
The Evaluation Process
According to information published on the clinic’s website, the treatment process begins with a thorough evaluation. Dr. Khan or Dr. Ryklin examines each patient’s medical history, reviews imaging studies such as MRI and X-rays, and identifies the specific source of nerve pain. This diagnostic approach ensures treatments target the root cause rather than merely addressing symptoms.
Stem Cell Administration
The practice partners with Amnio Technology, which produces amniotic tissue allografts processed in an American Association of Tissue Banks (AATB) accredited facility. Each batch undergoes testing to confirm it is free from transmittable diseases. The cells are sourced from a US-based Arizona company following FDA protocols regarding sterility and handling.
Dr. Daniel Ryklin describes the procedure on the clinic’s website: “Our regenerative treatments typically take between just 5-15 minutes to administer.” The minimally invasive nature of the treatment means patients can often resume normal activities quickly.
What Makes Amniotic Stem Cells Different
Arizona Pain and Spine Institute uses amniotic stem cells rather than requiring patients to undergo painful bone marrow or fat extraction procedures. According to information on the clinic’s website, amniotic cells are “packed with natural growth factors that promote tissue regeneration and reduce inflammation.” The amniotic membrane tissue contains amnion without chorion, which eliminates the risk of transplant rejection.
Clinical Research on Stem Cells for Neuropathic Pain
The scientific foundation for stem cell therapy in neuropathy treatment continues to strengthen through ongoing research.
Meta-Analysis Findings
A comprehensive systematic review published in Stem Cell Research & Therapy in November 2024 analyzed seven controlled human trials examining stem cell therapy for diabetic peripheral neuropathy. The meta-analysis reviewed studies from databases including Ovid MEDLINE, Embase, Scopus, Web of Science Core Collection, and Cochrane CENTRAL through January 31, 2024.
The findings indicated statistically significant improvements in nerve function. Motor nerve conduction velocity showed weighted mean differences of 2.2 (95% CI 1.6–2.8), while sensory nerve conduction velocity improved with weighted mean differences of 1.9 (95% CI 1.1–2.6). The stem cell therapies studied included bone marrow-derived mononuclear cells and umbilical cord-derived mesenchymal stem cells, administered mainly through intramuscular transplantation.
Preclinical Evidence
Research from Cleveland Clinic, as reported by Dr. Jianguo Cheng, Professor of Anesthesiology and Director of the Cleveland Clinic Multidisciplinary Pain Medicine Fellowship Program, has demonstrated the effectiveness of MSC transplantation in animal models. According to Cleveland Clinic’s published research summaries, MSC transplantation significantly reduced pain sensitivity in animals with nerve injuries. The cells produced immune modulatory and anti-inflammatory effects while promoting sensory nerve repair.
Dr. Cheng’s research indicates these cells demonstrate a unique ability to migrate to injury sites. “For reasons we do not yet fully understand, these cells have the ability to migrate to the injury site to promote repair of the injured nerve fibers,” Dr. Cheng states in the Cleveland Clinic publication.
Clinical Trial Landscape
According to records on ClinicalTrials.gov summarized in a July 2024 Stem Cell Research & Therapy article, 28 interventional clinical trials of MSC-based analgesia therapy with pain as an outcome measure were registered as of April 14, 2024. Discogenic pain and joint-related pain comprised the majority of these trials, with studies also addressing refractory migraine and trigeminal neuralgia.
Measurable Outcomes and Patient Experiences
Patient testimonials published on Arizona Pain and Spine Institute’s website provide insight into real-world outcomes with regenerative treatments.
Documented Patient Results
One patient testimonial describes the progression from unsuccessful traditional treatments to stem cell therapy: “I had been suffering from terrible neck and back/sciatica pain for years. I went to see Dr. Ryklin, and we started with epidurals, then ablation, and nothing had worked so far. Dr. Ryklin suggested Stem Cells for the next process, and I have been at least 90% better and out of pain for the last two years! It’s incredible how well the Stem Cells worked, and almost immediately!”
Another testimonial states: “Having had positive results with stem cell regenerative injections, I feel very confident encouraging this alternative to more invasive procedures. After years of pain, I can now say my neck and knee pain are nearly 100% gone, and my recently treated back is improving daily.”
A third patient reports: “After just one stem cell injection, most of my symptoms vanished, and I was so relieved of my pain. I’d like to thank Dr. Ryklin and his team for introducing me to stem cell therapy and restoring my quality of life!”
Timeline Considerations
Dr. Ryklin notes on the clinic’s website that “many of our patients report reliable results within just weeks of treatment.” The regenerative nature of the therapy means results may continue to develop over time as tissue healing progresses. However, individual outcomes vary based on factors including the severity and type of neuropathy, overall health status, and adherence to post-treatment recommendations.
The Arizona Neuropathy Landscape
Understanding the local context helps illustrate why regenerative treatments matter for Arizona residents.
Diabetes and Neuropathy Connection
The 2025 Arizona Diabetes Action Plan and Report released by the Arizona Department of Health Services documents that over 615,000 adults in Arizona live with diabetes, while nearly 2 million have prediabetes. The report notes that close to 90% of those with prediabetes are unaware of their condition.
According to data from the American Diabetes Association and National Institutes of Health, diabetic peripheral neuropathy affects approximately 50% of adults with diabetes during their lifetime. The NCBI StatPearls database indicates that roughly 70% of diabetics in the United States have some form of nerve damage that can result in neuropathy symptoms.
The Arizona Department of Health Services identifies nerve damage as a significant complication of improperly managed diabetes, alongside stroke, kidney disease, heart disease, blindness, and lower limb amputation.
Economic Burden
The 2025 Arizona Diabetes Action Plan documents that diabetes costs Arizona $6.7 billion annually. This includes $5.1 billion in direct medical costs and $1.6 billion in indirect costs related to absenteeism, reduced work productivity, early disability, and mortality.
Practical Implementation: What to Expect
Arizona Pain and Spine Institute has established clear protocols for patients considering stem cell therapy for neuropathy.
Consultation Process
The first step involves scheduling a consultation with Dr. Khan or Dr. Ryklin. During this appointment, the physician reviews medical history, conducts a physical examination, and evaluates diagnostic imaging. This comprehensive assessment determines whether stem cell therapy is appropriate for the individual patient’s condition.
Treatment Day
On treatment day, the procedure occurs in an outpatient setting without the need for a hospital stay. Using precise imaging guidance when necessary, the physician injects the amniotic stem cells into the areas causing nerve pain. The entire injection process takes approximately 5-15 minutes according to information on the clinic’s website.
Post-Treatment Period
Following the procedure, patients typically experience minimal downtime. The clinic’s website indicates most patients can carry out day-to-day activities within a few days and may see improvement in their condition within weeks. The practice emphasizes that stem cell therapy works by supporting the body’s natural healing processes, meaning results develop progressively as tissue regeneration occurs.
Insurance and Cost Considerations
Arizona Pain and Spine Institute notes on their website that most insurance plans do not currently cover regenerative medicine treatments. The practice offers financing options and flexible payment plans to make treatment accessible. The clinic also offers a FastTrack cash-pay program for patients who want expedited access to appointments, imaging, and procedures.
Comparing Treatment Approaches

Traditional neuropathy treatments and regenerative approaches differ fundamentally in their mechanisms and goals.
Conventional Treatment Limitations
According to a December 2024 review published in Neuroscience on ScienceDirect, existing neuropathy pain therapies “do not restore nerve function but only provide limited symptomatic relief.” Current pharmacological options include tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants, and topical agents—all of which address symptoms rather than underlying nerve damage.
Regenerative Medicine Advantages
Stem cell therapy aims to address root causes by promoting tissue repair. Research published in Frontiers in Cell and Developmental Biology in February 2025 describes how mesenchymal stem cells play important functional roles in promoting injured nerve regeneration through multiple mechanisms including regulating inflammation, supporting neuronal survival, and encouraging axonal growth.
When Stem Cell Therapy May Be Appropriate
Arizona Pain and Spine Institute suggests stem cell therapy as an option for patients who have not found success with conventional treatments or who want to avoid surgical interventions. The practice’s multidisciplinary approach often combines stem cell therapy with other pain management techniques such as nerve blocks, radiofrequency ablation, and physical therapy referrals.
Conclusion
The November 2024 meta-analysis demonstrating significant improvements in nerve conduction velocity among neuropathy patients represents meaningful progress in regenerative medicine. For Arizona’s 615,000-plus diabetes patients and the many others experiencing neuropathy from various causes, stem cell therapy offers a treatment approach that addresses underlying tissue damage rather than merely masking symptoms.
Arizona Pain and Spine Institute’s approach—combining AATB-accredited amniotic stem cells with individualized treatment planning from board-certified specialists—provides East Valley residents access to emerging regenerative treatments. Patient testimonials document outcomes including substantial pain reduction and functional improvement, though individual results vary.
Next Step: Schedule a consultation with Arizona Pain and Spine Institute at (480) 986-7246 to determine whether stem cell therapy for neuropathy is appropriate for your specific condition. The practice maintains locations in Mesa, Gilbert, and Queen Creek to serve patients throughout the Phoenix metropolitan area.
Frequently Asked Questions
Q: How long does stem cell therapy for neuropathy take to show results?
A: According to Arizona Pain and Spine Institute, many patients notice improvement within weeks of treatment, with some experiencing relief almost immediately. Results continue developing over several months as tissue regeneration progresses.
Q: Is stem cell therapy for neuropathy covered by insurance?
A: Most insurance plans currently do not cover regenerative medicine treatments. Arizona Pain and Spine Institute offers financing options and flexible payment plans to help make treatment accessible.
Q: What types of neuropathy can stem cell therapy treat?
A: Research has examined stem cell therapy for diabetic peripheral neuropathy, chemotherapy-induced neuropathy, and neuropathy from nerve injuries. A consultation determines whether specific conditions are appropriate for treatment.
Q: How is the stem cell procedure performed?
A: The procedure involves injecting amniotic stem cells into affected areas, typically taking 5-15 minutes. It’s performed in an outpatient setting with no hospital stay required.
Q: Are there risks associated with stem cell therapy for neuropathy?
A: Arizona Pain and Spine Institute uses cells processed in an AATB-accredited facility and tested for transmittable diseases. The November 2024 meta-analysis in Stem Cell Research & Therapy reported no serious adverse effects in the reviewed clinical trials.
Disclaimer
This article references publicly available information from Arizona Pain and Spine Institute, Arizona Department of Health Services, Stem Cell Research & Therapy journal, Cleveland Clinic, National Institutes of Health, and American Diabetes Association including official documentation, press releases, peer-reviewed research, and published case studies dated 2019-2025. All metrics and quotes are from documented sources. Results described are specific to the research studies and individual patients mentioned and may vary based on individual health conditions, severity of neuropathy, and treatment approach. For current information about services offered, consult Arizona Pain and Spine Institute directly at gotpainarizona.com. This content does not constitute medical advice. Consult a qualified healthcare provider for diagnosis and treatment recommendations.